
5 Things to Do to Help the Future of Campus Placements in IT
21/06/2024
What are the Different Types of Recruitment?
25/06/2024Did you know that the average investment in recruiting one patient for a clinical trial is estimated to be around $6,500? But that’s just the tip of the iceberg. The real struggle starts after, when clinical trial co-ordinators must retain the patients for the duration of the study. And it’s an ever-known fact that more often than not, patients drop out of the trial incredibly early, and this is one of the significant concerns in the industry.
However, pharma sponsors and clinical research organisations have been actively finding ways to improve the clinical trial patient retention rates with the help of patient support programs.
How can an effective PSP (Patient Support Programs) help with the clinical research?
Before getting into the measures of retaining patients, it’s important to understand why patients drop out of the trial so often? What are the barriers preventing them for active participation? Is it missed work? Complex travel arrangements, unrest due to a lot of time away from home and family? Delayed reimbursements?
It’s all of them.
There are several steps and measures pharma companies can take to improve their patient retention rates, leading to successful drug trials.
Constant Patient/representative involvement during the development of medicines
Who better than a patient to give feedback on the effectiveness of a drug trial that’s being done on them? From pre-clinical studies to launch and beyond, for the entire span during which the medicine is available to the patients, only they can provide you with the most valuable insights.
A study by The Economist Intelligence Unit found drugs developed with patient-centric processes were:
- 20% more likely to launch than drugs without a patient centric approach
- Quicker to recruit 100 participants than the others which took about 6-11 months
Patients, with the other stakeholders, play a key role in determining the effectiveness of the medicine, as ultimately, it’s them who may or may not reap the benefits. By improving patient involvement, there can be greater innovation of medicines that deliver impactful outcomes. Designing the trials and protocols around the patient’s need and circumstances will allow more patients to participate in clinical trials with a far less dropout rate.
Assist patients circumstances to favour the clinical trials
Its ultimately the patients who can bring success to a clinical trial. So, making them the centre of attention and prioritising them makes sense socially, financially and clinically. Some of the ways to improve patient experience are-
- Clinical trial teams can assist them with study logistics
- Implement wearable monitors, video appointments to reduce physical visits
- Establish communication and keep them updated
- Patient co-ordinators must work directly with patients and caregivers to ensure comfort and safety
- Patient co-ordinators must establish a trusting relationship with patients and caregivers.
- Patient support providers can also alleviate the complexities of multi-country rare disease trials, which often require extensive travel, visas, relocation, language translation, housing, and transport of medically vulnerable and paediatric patients.
Open and on-going communication with the patients keeps them updated
Having open communication lines between the clinical trial patients can go a long way in increasing patient retention rates. One-on-one discussions help patients expect the scope and commitment involved in trial participation while demonstrating the level of support provided throughout the study to overcome potential challenges.
Access to information for every patient
The internet and digital media resources have made it so easy for patients to access information and exchange experiences. This helps patients gain knowledge about their disease and treatment options. Some portals even offer tracking opportunities of individual’s health parameters. And this is what must be done in clinical trials. An informed patient is an empowered patient.
Educating patients about the drug development for a meaningful input
For meaningful patient input in drug development and evaluation, stakeholders must provide not only information but also specific knowledge, actively contributing toward this goal.
A good example is the European Patients’ Academy on Therapeutic Innovation (EUPATI), a collaborative public-private partnership project of 30 organizations that is funded by the Innovative Medicines Initiative. It was formed to increase the number and capabilities of patients and related organizations to advise on drug development.
Partner with patient support program services provider
Clinical trial sponsors can ensure higher retention rates by focusing on the needs of the patients involved. Ultimately, patient-centric clinical trials expedite the market arrival of new therapies while supporting the pharmaceutical industry’s primary goal: caring for the health and well-being of patients worldwide.
Case Study
A review of a multi-year clinical study underscores the significant impact of patient care coordinators on trial outcomes. This study is researching a new treatment for Duchenne Muscular Dystrophy (DMD), a rare progressive muscle-wasting disease primarily affecting boys.
In rare diseases like DMD, patient populations are typically small and widely dispersed across the globe. The DMD study spans 35 trial sites across nine countries. Over five years, 18 patient care coordinators have supported more than 170 trial patients and their caregivers, including arranging cross-border travel and accommodations.
How do the patient co-ordinators support the patient?
The coordinators ensure patients feel comfortable in new cultural environments by
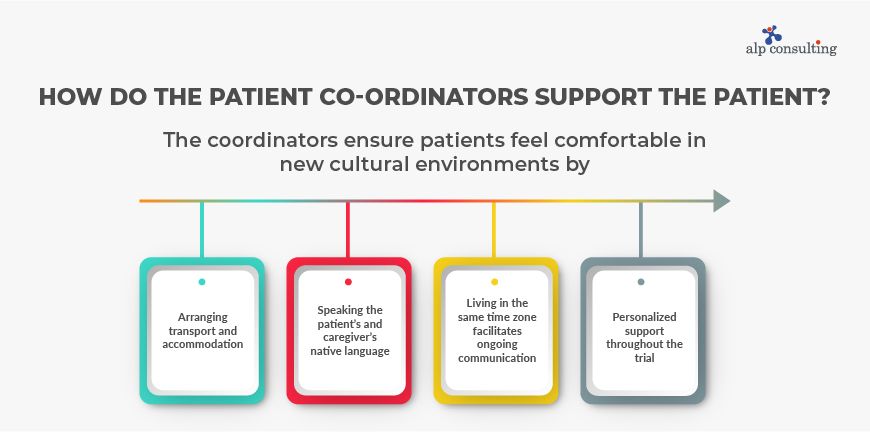
- arranging transport and accommodation
- speaking the patient’s and caregiver’s native language
- Living in the same time zone facilitates ongoing communication
- personalized support throughout the trial.
Travel logistics have included airplane, rail, and wheelchair-accessible car services. Patient care coordinators have booked over 200 airline flights, 250 ground transportation trips, and 530 hotel reservations. More than 1,300 reimbursements have been processed following site visits.
To expect the needs and travel preferences of DMD patients, who often need wheelchairs, coordinators ensure ADA-compliant hotel rooms, accessible bathrooms, and suitable living spaces. They also take responsibility of managing dietary needs, providing safe foods to accommodate patients’ difficulties with chewing and swallowing and adhering to strict dietary guidelines. As medical needs evolve, coordinators continue to adjust logistics accordingly.
By alleviating the emotional, financial, and logistical burdens of clinical trial participation, coordinators enable patients, caregivers, and trial teams to focus on developing life-changing treatments. Although the trial is ongoing, it stays on schedule and within budget, supported by a patient retention rate that exceeds industry benchmarks.
Conclusion
PSPs (Patient Support Programs) play a crucial role in accelerating clinical research by addressing the unique needs faced by the participants. They are especially useful in improving patient retention rates as they provide support like logistical aid, emotional support- and personalized care. By alleviating the burdens associated with trial participation, PSPs enable patients and caregivers to focus on treatment, leading to more correct and reliable trial outcomes.
Contact Us For Business Enquiry

Dr. Neha Joshi
Dr. Neha Joshi is the Business Head at Alp Consulting Ltd., bringing over 15 years of diversified experience in operations management, business strategy, and client excellence across healthcare and consulting sectors. Her expertise lies in driving operational transformation, enhancing customer experience, and building scalable business processes. Having held key leadership roles at Rivaara Labs, Suburban Diagnostics, and Portea, Neha combines strategic insight with execution excellence to deliver impactful, sustainable growth.




